The Photosynthetic Apparatus
Photosystem 2
Photosystem 2 is the largest protein complex found in nature and due to its unique function is also one the most studied (See the reviews by Hankamer et al., 1997, Barber and Kühlbrandt, 1999, and Diner and Rappaport, 2002).
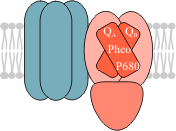
The holocomplex of PS2 is consisted of some 25 polypeptides embedding all pigments and cofactors necessary to oxidise water and drive the photo-induced electron transport to plastoquinone. The crystal structure of PS2 was determined by Zouni et al. (2001). Briefly, PS2 is comprised of a core complex with its core antenna, an oxygen-evolving complex (OEC) and a light-harvesting complex (LHC2) (Fig. 3). Because LHC2 can be detached from the rest of PS2 and migrates independently, it is sometimes regarded as the fith supercomplex of the thylakoid membrane.
The light-harvesting complex 2 is the most abundant complex of the thylakoid membrane and binds more than half of all chlorophylls (See Kühlbrandt, 1994). Each LHC2 subunit is formed by three homologous proteins and binds 7 chlorophyll a molecules, 5 chlorophyll b molecules and a few carotenoids (Peter and Thornber 1991). Upon absorption of a light quantum the chlorophyll molecules are excited and the light energy is thus passed to their electrons. The chlorophylls are specially arranged in the protein bed so that the excitation energy can instantly migrate from one molecule to another and from the light-harvesting antenna to the PS2 core complex (for the physical mechanisms of energy transfer in the photosynthetic antennae, see van Grondelle et al., 1994 , van Amerongen et al., 2000, Green and Parson, 2001).
The PS2 core complex contains an inner antenna, which is somewhat similar in structure to the LHC2 subunits, and the reaction centre, where the primary steps of light-energy conversion are carried out. The essential cofactors of the PS2 reaction centre are the reaction centre chlorophylls, P680, the primary electron acceptor, pheophytin, and two quinone molecules, QA and QB. They are bound to two transmembrane proteins, D1 and D2. The light energy collected by the light-harvesting complex is delivered via the inner antenna to the reaction center chlorophyll, P680. The excited electron of P680* can be donated to the pheophytin, which is the primary photochemical reaction. The electron is passed from pheophytin to QA and from this point onwards there is a stable charge separation. The next electron acceptor, QB has the same chemical structure as QA but it can accept two electrons instead of one. The quinone acceptors are found on the stromal side of PS2, so the double-reduced QB2− takes two protons from the stroma and is detached from the PS2 complex as a freely-moving plastoqiunole (PQ.H2), while the QB site gets occupied by another (oxidised) plastoquinone molecule.
The chlorophyll cation-radical P680+ is a highly potent oxidiser and can extract electrons from water. The oxidation of water is carried out in the oxygen-evolving complex, located on the lumenal side of PS2. For the mechanism of water oxidation, see e.g. Renger (2001).